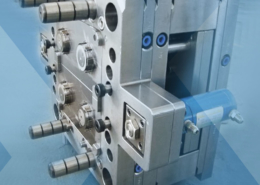
The role of injection moulding in medical device ... - medical injection mouldin
Author:gly Date: 2024-10-15
We continuously work to ensure that we meet all quality and regulatory requirements throughout your entire product life-cycle.
We are ISO 13485: 2016 certified. Our Quality Management System (QMS) is designed to meet all of our customers’ expectations and documentation requirements and we will work with you to address any additional requirements.
Polypropylene Beakers are resistant to most commonly used laboratory reagents, including a variety of strong acids, bases, and organic solvents, these high quality polypropylene beakers are the premier choice for scientific laboratory applications. All beakers are transparent for clear content visibility and graduated for easily estimating volumes. Molded-in pouring spouts, and a rolled lip, for a dripless working environment. Autoclavable to 121ºC ( 248ºF).
BMP Medical Cytology Funnels for the Shandon CytoSpin® Centrifuge BMP Medical Cytology Funnels are the functional equivalent of the Thermo Shandon CytoFunnel®. Fully compatible with the Shandon CytoSpin Centrifuge, BMP Medical Cytology Funnels can be used to deposit a thin layer of cells in a clearly defined area of a microscope slide. The filter card absorbs any excess fluid. Operation is identical to those supplied by Shandon and easy-to-follow instructions are included in each pack. All products sold in case quantities only.
The Hemodrop is designed to reduce the risk of exposure to bloodborne pathogens in the Hematology laboratory. To prepare a manual differential smear, a drop of blood needs to be dispensed onto a glass slide from the specimen tube. Simply puncture the rubber stopper with the Hemodrop, and then invert and press gently onto the slide. A single drop of blood will be dispensed and the slide is ready. No more removing the stopper and manually putting a drop on the slide to test blood.
These precision molded centrifuge bottles are available in polycarbonate or polypropylene. For low speed applications the bottles provide excellent value for large volume/large batch centrifugation procedures. For high-speed work, the easy-to-use Noryl® (Noryl® is a registered trademark of General Electric Company) sealing caps with silicone O-rings enable leak-free processing at high centrifugal forces over long durations. Bottles must be filled to 80% of capacity.
BMP Medical, a manufacturer of diagnostic products, has a full line of urinalysis disposables. We offer the components separately, or in various kit sizes and configurations. The line is built around our 10-chambered Decislide which is made out of optically clear acrylic. It can take 10 individual samples on the same slide eliminating the need for a new slide for each sample.
Step one is to establish the correct Intellectual Properties register rights for your product. Once that is complete, you are ready to engage with BMP Medical as your trusted manufacturing partner. We understand your concerns when it comes to protecting your IP. We will work closely with you on developing a signed nondisclosure agreement that states your organization owns the IP.
Our workforce boasts a high degree of experience and training in the manufacturing of plastic medical devices and components for OEM partners. We will work together with you on developing a personalized and effective quality plan and assurance program with routine audits that meets all of your quality, traceability and consistency requirements. We’ll be your trusted long-term partner. BMP Medical employees are trained with an emphasis on the “pursuit of perfection” in all we do.
Fortify is the leader in molding high-performance plastics. Fortify’s medical device injection molding services can mold medical device components in high-quality, medical-grade plastics, so you can mold parts in end-use material without high tooling costs.

BMP Medical is a quality manufacturer of plastic consumables and plastic products for research and medical diagnostic laboratories. Our products are manufactured to the highest quality standards. We offer a wide selection of products sold through the world’s premier laboratory distributors.
Medical-grade plastics are available in injection molding pellets, but these plastics are not widely available in 3D printing. With Fortify’s injection molding services, you can develop prototypes at 3D printing speeds with validated materials.
We are a privately held company that has been in business for over 45 years. We use state-of-the-art molding techniques and machinery to produce varying-sized components with very tight specifications. We also offer design consultation, engineering, drawing, R&D, tooling, mold development, process automation, and statistical process control. Our facility is equipped with a Class 8 cleanroom for manufacturing, assembly, and packaging needs.
For specimen collection and transport, this system provides disposable products for the convenient collection, delivery, and testing of urine specimens. A plastic cup is provided for specimen collection and the pressure sensitive patient identification labels have ample writing space. The lightweight transport rack is small and easy to carry yet provides space for up to 20 tubes with labels and caps. The tubes are made of sturdy polystyrene and are safe for centrifuging. The flared top permits the use of a midget urinometer ensuring a tight cap fit.

Efficiency and precision are paramount in the medical device manufacturing industry. As technology advances and regulatory requirements become more stringent, the need for streamlining the assembly process has never been more
At BMP Medical, a leader in contract manufacturing for medical devices, we prioritize fostering a work environment that promotes growth, safety, and inclusivity for all employees. Our recent initiatives reflect our dedication to continuous improvement and workforce...
BMP Medical is a leading plastic manufacturing of medium- to high-volume medical devices and components. Our global state-of-the-art facility located in Sterling, Massachusetts, offers product design and development, R&D tooling, custom cleanroom assembly and packaging, Class 7 assembly, and Class 8 cleanroom manufacturing. Whether you have a new device concept, an approved design, or a design transfer, we’ll work with you to provide the best injection molding solution.
Our 80,000 sq. ft. plastics manufacturing facility is based in North America and headquartered in Sterling, Massachusetts.
BMP Medical is a family-owned business with nearly one hundred employees. We foster strong, long-term relationships to develop tailored plastic manufactured solutions for your medical device and component needs. You will always experience a one-on-one personal touch with a complete team approach when interacting with BMP Medical. Whether you are reviewing a quotation with our sales representatives or discussing a prototype with our engineers, you will always experience a friendly, knowledgeable, and extremely dedicated individual. We work endlessly to provide optimal value to our customers, and take pride in our ability to be agile to your evolving needs. Get to know BMP Medical!
In today's healthcare landscape, precision, reliability, efficiency and quality are paramount. Medical injection molding companies play a crucial role in meeting these demands, especially in the development of life-enhancing medical devices, diagnostic tools, and...
These high-quality non-graduated lab containers are designed for the collection, transport, and storage of specimens and samples. Tight fitting lids prevent leaks and odors. Ideal for storing biologically hazardous samples for terminal autoclaving before disposal. Molded from various materials. The stackable containers are resistant to freezing and boiling.

When collaborating with BMP Medical, you will experience very robust quality assurance standards. BMP Medical is ISO 13485:2016 certified, fully FDA compliant, and cGMP. Our continuous improvement approach provides OEMs superior quality and better management oversight of their suppliers. Ask us why BMP Medical became the first MedAccred certified injection molding company within the MedTech industry.
Failure to use the correct rotor cushion or adapter will shorten the life of the bottle and may prevent achievement of the maximum rated RCF and/or bottle failure.
We offer two lines of tissue grinders for the clinical lab and research lab. The standard product is all plastic and is designed to grind softer tissues such as liver and spleen. The Ultra tissue grinder line has a vitrified tip and is designed for tougher material such as muscle or bone. Both lines are delivered sterile sealed Tyvek® (Tyvek® is a registered trademark of DuPont) package.
Centrifuge bottles may be autoclaved at 121ºC at 15 psi for 20 minutes. Caps should be placed on the bottles without engaging the threads. Bottles are qualified to the rated g-forces using Sorvall® (Sorvall® is a registered trademark of Kendro Laboratory Products) and Fiberlite® (Fiberlite® is a registered trademark of Piramoon Technologies Inc.) high-speed rotors, tested at 20ºC, with bottles filled to 80% of capacity.
Medical device development is a high-risk product development cycle where many products cost thousands of dollars to develop without ever going to market. By using faster, cost-effective injection molding for developing medical device components, the risk of innovation and iteration is eliminated.
GETTING A QUOTE WITH LK-MOULD IS FREE AND SIMPLE.
FIND MORE OF OUR SERVICES:


Plastic Molding
Rapid Prototyping

Pressure Die Casting

Parts Assembly



