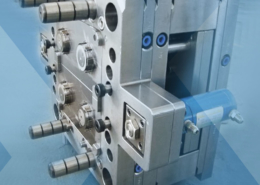
Plastic injection molding dies using hybrid additively manufactured 420/CX
Author:gly Date: 2024-09-30

Lest this week’s news about FDA cautioning against the use of plastic syringes made in China raise concerns of a possible shortage, BD announced today that it is increasing its US production of syringes.


Plasteurope.com is a business information platform for the European plastics industry. It is part of KI Kunststoff Information and PIE Plastics Information Europe, one of the leading content providers for the European plastics industry. We offer daily updated business news and reports, in-depth market analysis, polymer prices and other services for the international plastics industry, including a suppliers guide, career opportunities, a trade name directory and videos.
"BD has the capacity to support additional syringe demand and is further increasing US production to help ensure continuity of patient care,” said BD Medication Delivery Solutions President Eric Borin in today’s announcement. “Since the initial FDA safety communication in November, BD has increased domestic manufacturing of syringes in our Nebraska and Connecticut facilities to respond to customer needs.”
The medtech giant is ramping up domestic syringe production in light of FDA’s recommendation to transition away from plastic syringes made in China.
Noting that no BD syringes are mentioned in FDA’s safety communication, which recommends that users in the United States transition away from China-made syringes, Borin stressed that ensuring the safety and quality of its products is a priority at the 125-year-old medical device company. Notably, BD manufactured two billion additional syringes and needles to support the global response to the COVID-19 pandemic.
© 2001-2024 Plasteurope.com | Imprint | Privacy | Cookie settings Plasteurope.com is a business information platform for the European plastics industry. It is part of KI Kunststoff Information and PIE Plastics Information Europe, one of the leading content providers for the European plastics industry. We offer daily updated business news and reports, in-depth market analysis, polymer prices and other services for the international plastics industry, including a suppliers guide, career opportunities, a trade name directory and videos. News | Polymer Prices | Material Databases | Plastics Exchange | Suppliers Guide | Jobs | Register | Advertising PIE – Plastics Information Europe | KI – Kunststoff Information | KunststoffWeb | Plastics Material Exchange | Polyglobe | K-Profi
FDA began evaluating medical plastic syringes made in China near the end of last year when it learned of quality issues such as leakage or breakage and the potential that the faulty syringes may deliver incorrect dosages of medication. This week FDA reported in an update that the quality issues were more widespread than originally known. It sent warning letters to some China-based manufacturers of plastic syringes as well as US-based Medline Industries and Sol-Millennium, which market the syringes domestically.
Editor in chief of PlasticsToday since 2015, Norbert Sparrow has more than 30 years of editorial experience in business-to-business media. He studied journalism at the Centre Universitaire d'Etudes du Journalisme in Strasbourg, France, where he earned a master's degree.
One of the largest medical device companies in the world, BD also claims to be one of the largest users of plastic injection molded products in the world. At the groundbreaking of BD’s plant in Columbus, NE, James Borzi, executive vice president and chief supply chain officer at the time, said that the new facility will “centralize a majority of BD’s North American plastic molding production and will support multiple business units in the United States and around the world.” BD operates three other facilities in Nebraska.
GETTING A QUOTE WITH LK-MOULD IS FREE AND SIMPLE.
FIND MORE OF OUR SERVICES:


Plastic Molding
Rapid Prototyping

Pressure Die Casting

Parts Assembly



