
Renewable-Energy Powered Storage System Slashes Roto-Molder’s Fuel Costs
Author:gly Date: 2024-09-30
A key feature of the device is the tip of the applicator, which is made of polyurethane foam. The gas flows through this foam and freezes it. The user places the tip onto the wart, transferring the cold to the wart. The soft foam ensures that contact with the wart is ideal because when it heats up it makes excellent surface contact.
“The Pixie pen embodies the fusion of our knowledge in the field of plastics engineering and polymer processing and the knowledge of an external industry expert, in this case in the field of medical devices,” said Jan Claeys, Account Manager at Quadrant CMS.
The Pixie pen is automatically assembled from 14 components. Of those, Quadrant CMS injection molds six plastic components using five different engineering plastics.
PharmiWeb.com is Europe's leading industry-sponsored portal for the Pharmaceutical sector, providing the latest jobs, news, features and events listings.The information provided on PharmiWeb.com is designed to support, not replace, the relationship that exists between a patient/site visitor and his/her physician. This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
The Pixie pen is CE marked and approved for use in the European Union as a Class IIa medical device. All processes at Oystershell and Quadrant have been accredited through external audits under standards equivalent to ISO 13485. Biological and clinical studies indicate that the Pixie pen is superior to various other available methods for the treatment of warts, according to the companies.

Key components of the device must withstand high pressures, remain gas tight and accommodate an ultra-low application temperature (-80°C) as the nitrous oxide expands from liquid to gas and freezes the targeted wart. Current products for freezing warts at home involve canisters with a dangerous mixture of liquefied propane gas and dimethylether. The Pixie pen, which can be used for up to eight treatments, has an attractive price point and will revolutionize the current market, says Bart Rossel, CSO at Oystershell.
managing a total of 16 equipment and parts suppliers and the combination of 14 components (including eight sourced from outside and developed specifically for the Pixie);

> Recognize newcomers with potentially strong product portfolios and devise effective counter-strategies to acquire a competitive edge.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
The first phase of the project involved an intensive study by a special Quadrant CMS development team, working in close collaboration with medical researchers at Oystershell and experts from external suppliers. The study involved input on engineering, production, quality control, purchasing and sub-assembly.
> To increase and grow business potential and reach, develop and plan licencing and licencing strategies by finding possible partners with the most appealing projects.
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
Quadrant Creative Moulding & Systems (Quadrant CMS; Tielt, Belgium) collaborated with pharmaceutical company Oystershell Laboratories (Drongen, Belgium) in the development of a safe, easy-to-use wart removal device. The Pixie pen, which doses small amounts of nitrous oxide onto the skin from a pressurized container, is automatically assembled from 14 components. Of those, Quadrant CMS injection molds six plastic components using five different engineering plastics, including acetal, polycarbonate, nylon, PMMA and SAN.
> Suitable for providing dependable and high-quality data and analysis to assist your internal and external presentations.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
PharmiWeb.com is Europe's leading industry-sponsored portal for the Pharmaceutical sector, providing the latest jobs, news, features and events listings.The information provided on PharmiWeb.com is designed to support, not replace, the relationship that exists between a patient/site visitor and his/her physician.
The Pixie pen began appearing in pharmacies in Belgium a few weeks ago. Oystershell has licensed the rights for the product to a top-five global pharmaceutical company for the whole of Europe; North American and Asian markets will follow soon.
Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
Prototype dispensers were assembled from machined plastic parts that were made in collaboration with another Quadrant company, Quadrant Engineering Plastic Products, also in Tielt. Exactly one year after the start of the project, Quadrant produced the first pen using components it had injection molded on prototype tools.
Request Sample of the Report on HIV Injection Market Forecast 2033 https://www.alliedmarketresearch.com/request-sample/A312373
The growth of the HIV injection market is fueled by an increase in the number of individuals affected by HIV, greater awareness surrounding early diagnosis and treatment, and a rise in research and development initiatives. For example, as reported by the World Health Organization (WHO) in July 2023, HIV continues to be a significant global public health concern, with advanced HIV disease remaining a persistent issue in HIV response efforts. According to the same source, approximately 39.0 million people were living with HIV by the end of 2022, and around 1.3 million people acquired HIV in that same year.
During an economic downturn, both individuals and governments may tighten their budgets, resulting in decreased healthcare spending. This could limit patients’ access to and affordability of HIV injections, potentially reducing demand for such treatments. However, the increasing prevalence of HIV infections sustains demand for therapeutic drugs, even during a recession. Consequently, there may be a moderately positive impact on the demand for HIV injections, driven by the rising need for treatment amidst economic challenges.
Following prototyping, six injection molds were built for high-series production and the assembly process was finalized. Assembly involves several semi-automated quality control checks done under medical-device-regulated conditions and numerous automated quality checks.
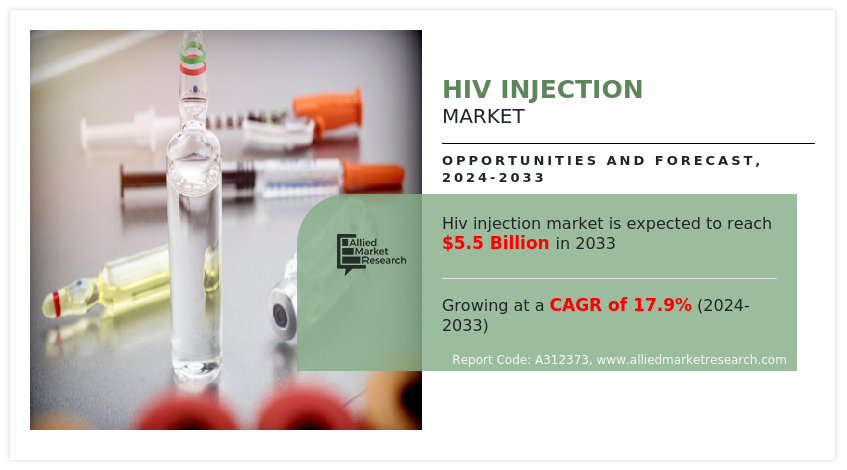
Allied Market Research published a report, titled, “HIV Injection Market by Distribution Channel (Hospital Pharmacy, Drug Stores and Retail Pharmacies, and Others): Global Opportunity Analysis and Industry Forecast, 2024-2033″. According to the report, the global HIV injection industry generated $1.0 billion in 2023, and is anticipated to generate $5.5 billion by 2033, witnessing a CAGR of 17.9% from 2024 to 2033.
GETTING A QUOTE WITH LK-MOULD IS FREE AND SIMPLE.
FIND MORE OF OUR SERVICES:


Plastic Molding

Rapid Prototyping

Pressure Die Casting

Parts Assembly



