Engel cautious about outlook for moulding machinery markets - engel injection mo
Author:gly Date: 2024-09-30
Lyumjev® and Humalog® are registered trademarks owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates.
INDIANAPOLIS, Aug. 16, 2021 /PRNewswire/ -- The U.S. Food and Drug Administration (FDA) has approved an expanded label for Eli Lilly and Company's (NYSE: LLY) rapid-acting insulin, Lyumjev® (insulin lispro-aabc injection) 100 units/mL indicated to improve glycemic control in adults with type 1 and type 2 diabetes, to include administration via continuous subcutaneous insulin infusion (CSII) with an insulin pump.
In the future, it won’t only be PET bottles that will serve as resource for recycling and reuse as rPET, but PET and rPET plastic cups as well.
Lilly Cautionary Statement Regarding Forward-Looking StatementsThis press release contains forward-looking statements (as that term is defined in the Private Securities Litigation Reform Act of 1995) about Lyumjev® (insulin lispro-aabc injection) and Humalog (insulin lispro injection) as treatments to improve glycemic control in adults with type 1 and type 2 diabetes and reflects Lilly's current beliefs and expectations. However, as with any pharmaceutical product or medical device, there are substantial risks and uncertainties in the process of drug research, development, and commercialization. Among other things, there is no guarantee that future study results will be consistent with study results to date, that Lyumjev will be commercially successful, or that the company will meet its anticipated timelines for the roll out of this medicine. For further discussion of these and other risks and uncertainties, see Lilly's most recent Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission. Except as required by law, Lilly undertakes no duty to update forward-looking statements to reflect events after the date of this release.
Read the Instructions for Use that come with your Lyumjev or Humalog. Be sure to take your Lyumjev or Humalog and check your blood sugar levels exactly as your doctor tells you to. Your doctor may tell you to change your dose because of illness, increased stress, or changes in your weight, diet, or physical activity level. He or she may also tell you to change the amount or time of your dose because of other medicines or different types of insulin you take.
The lightweight packaging made of recycled material that can be readily recycled is a model of an optimally sustainable packaging from environmental and regulatory aspects.
Lyumjev is available in the U.S. and in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps. People with diabetes should seek guidance from their healthcare providers and refer to the insulin pump maker instructions to see if Lyumjev U-100 can be used with their pump.
"Insulin pumps are an important delivery option for people with diabetes – many of whom struggle with high postmeal blood sugar levels," said Leonard Glass M.D., F.A.C.E., vice president of Medical Affairs, Lilly. "The expansion of the Lyumjev label to include use in an insulin pump provides a new and important choice for people with diabetes. It is an exciting development for pump users seeking to manage their blood sugar levels and reduce postmeal spikes."

Do not change the type of insulin you take or your dose, unless your doctor tells you to. This could cause low or high blood sugar, which could be serious.
To stay safe while taking your insulin, be sure to never inject Lyumjev U-200 in your vein, muscle, or with an insulin pump. Also be sure not to:
Lilly offers several solutions to help eligible people who need assistance accessing their Lilly insulin, including Lyumjev, at the Lilly Diabetes Solution Center at (833) 808-1234 and www.insulinaffordability.com. People who have commercial insurance can visit www.Lyumjev.com to access the Lyumjev Savings Card. Lyumjev is also included in the Lilly Insulin Value Program for people with commercial insurance or no insurance at all, as well as in the Seniors Savings Model for people in the Medicare Part D program—allowing them to fill their monthly prescription of Lyumjev for $35. Other restrictions may apply.
The use of 100% rPET results in up to a four-fold reduction in carbon dioxide equivalent emissions compared to virgin PP, according to data using the Ecoinvent v.3.9 database.
These are not all of the possible side effects. Tell your doctor if you have any side effects. You can report side effects at 1-800-FDA-1088 or www.fda.gov/medwatch.
About the PRONTO-PUMP-2 StudyPRONTO-PUMP-2 was a phase 3, 16-week, randomized (1:1), active controlled, double-blind treat-to-target multinational study that evaluated the efficacy and safety of Lyumjev versus Humalog (insulin lispro injection) 100 units/mL in 432 adult patients with type 1 diabetes (T1D) using insulin pumps. Following a 2-week lead-in period, patients were randomized in a double-blind manner to either Lyumjev (n=215) or Humalog (n=217), both 100 units/mL. Patients were instructed to deliver bolus doses 0-2 minutes before meals. The primary objective was noninferiority (noninferiority margin [NIM]=0.4% for A1C) of Lyumjev to Humalog on change in A1C from baseline to week 16. Key multiplicity-adjusted secondary objectives included superiority of Lyumjev to Humalog at week 16 for 1- and 2-hour postprandial glucose during a meal test and duration of time in range (70-180 mg/dL) during daytime and over a 24-hour period.1
If you are at risk of having severely low blood sugar, your doctor may prescribe a glucagon emergency kit. These are used when your blood sugar becomes too low and you are unable to take sugar by mouth. Glucagon helps your body release sugar into your bloodstream.
Please see Lyumjev Full Prescribing Information including Patient Prescribing Information Please see Humalog Full Prescribing Information including Patient Prescribing Information
Lyumjev, a novel formulation of insulin lispro developed to speed the absorption of insulin into the bloodstream and reduce A1C levels, was approved by the FDA in June 2020. As a rapid-acting mealtime insulin, Lyumjev helps control blood sugar levels after meals in adults with diabetes, similar to how natural insulin works after meals in people without diabetes.
View original content to download multimedia:https://www.prnewswire.com/news-releases/fda-approves-lyumjev-insulin-lispro-aabc-injection-100-unitsml-for-use-in-insulin-pumps-301355407.html
The approval was based on results from PRONTO-PUMP-2, a phase 3 treat-to-target study that confirmed the efficacy and safety of Lyumjev when used in insulin pumps in adults with type 1 diabetes. The study met the primary endpoint of noninferior A1C reduction from baseline to week 16 compared to Humalog. Lyumjev demonstrated superior reduction in blood glucose spikes at both one and two hours after a test meal compared to Humalog.1
This summary provides basic information about Lyumjev and Humalog. It does not include all information known about these medicines. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking with your doctor. Be sure to talk to your doctor or other healthcare provider about your insulin lispro product and how to take it. Your doctor is the best person to help you decide if these medicines are right for you.
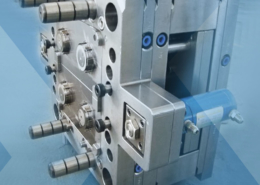
After an intensive development phase, Greiner Packaging has achieved a breakthrough in cooperation with injection molding machine maker Engel and mold maker Brink: Thin-walled cups made of recycled PET can now also be produced using injection molding technology. Suitable for filling lines, sealable and offering considerable carbon dioxide savings potential, the breakthrough opens new opportunities for packaging designed for a circular economy.
Do not use a syringe to remove Lyumjev or Humalog from your prefilled pen. This can cause you to take too much insulin. Taking too much insulin can lead to severe low blood sugar. This may result in seizures or death.
You can inject your insulin dose yourself, or you can have a trained caregiver inject it for you. Make sure you or your caregiver:
Sebastian Diensthuber, Greiner Packaging’s global product group manager, is thrilled by the innovation. "When developing the thin-walled, injection-molded cups made of PET, it was important for us to develop a solution that was not only innovative but also able to withstand our customers' industrial requirements. The cups we developed together with Brink and Engel are designed in such a way that a transition to the new generation of cups is possible, both in filling and sealing”.
About DiabetesApproximately 34 million Americans2 (just over 1 in 10) and an estimated 463 million adults worldwide3 have diabetes. Type 2 diabetes is the most common type internationally, accounting for an estimated 90 to 95 percent of all diabetes cases in the United States alone3. Diabetes is a chronic disease that occurs when the body does not properly produce or use the hormone insulin.
Lyumjev and Humalog may cause serious side effects. Some of these can lead to death. The possible serious side effects are:
About Lilly DiabetesLilly has been a global leader in diabetes care since 1923, when we introduced the world's first commercial insulin. Today we are building upon this heritage by working to meet the diverse needs of people with diabetes and those who care for them. Through research, collaboration and quality manufacturing we strive to make life better for people affected by diabetes and related conditions. We work to deliver breakthrough outcomes through innovative solutions—from medicines and technologies to support programs and more. For the latest updates, visit http://www.lillydiabetes.com/ or follow us on Twitter: @LillyDiabetes and Facebook: LillyDiabetesUS.
About Eli Lilly and CompanyLilly is a global health care leader that unites caring with discovery to create medicines that make life better for people around the world. We were founded more than a century ago by a man committed to creating high-quality medicines that meet real needs, and today we remain true to that mission in all our work. Across the globe, Lilly employees work to discover and bring life-changing medicines to those who need them, improve the understanding and management of disease, and give back to communities through philanthropy and volunteerism. To learn more about Lilly, please visit us at lilly.com and lilly.com/newsroom. P-LLY
Greiner Packaging, Engel, and Brink combine forces to develop thin-walled injection-molded recycled PET (rPET) cups designed for a circular economy.

GETTING A QUOTE WITH LK-MOULD IS FREE AND SIMPLE.
FIND MORE OF OUR SERVICES:


Plastic Molding
Rapid Prototyping

Pressure Die Casting

Parts Assembly



