Comar Expands Internationally with Acquisition of European Medical and
Author:gly Date: 2024-09-30
In this collection of content, we provide expert advice on welding from some of the leading authorities in the field, with tips on such matters as controls, as well as insights on how to solve common problems in welding.
Mold maintenance is critical, and with this collection of content we’ve bundled some of the very best advice we’ve published on repairing, maintaining, evaluating and even hanging molds on injection molding machines.
While the major correction in PP prices was finally underway, generally stable pricing was anticipated for the other four commodity resins.
This month’s resin pricing report includes PT’s quarterly check-in on select engineering resins, including nylon 6 and 66.
Introduced by Zeiger and Spark Industries at the PTXPO, the nozzle is designed for maximum heat transfer and uniformity with a continuous taper for self cleaning.
Chromatic will be demonstrating 3D printed parts made from ChromaMotive and its other material offerings at the International Elastomer Conference being held this week in Cleveland.
9T Labs continues to enhance the efficiency of its technology, which produces composite parts with intentionally oriented fibers.


Join KraussMaffei for an insightful webinar designed for industry professionals, engineers and anyone interested in the manufacturing processes of PVC pipes. This session will provide a comprehensive understanding of the technology behind the production of high-quality PVC pipes: from raw material preparation to final product testing. Agenda: Introduction to PVC extrusion: overview of the basic principles of PVC pipe extrusion — including the process of melting and shaping PVC resin into pipe forms Equipment and machinery: detailed explanation of the key equipment involved — such as extruders, dies and cooling systems — and their roles in the extrusion process Process parameters: insight into the critical process parameters like temperature, pressure and cooling rates that influence the quality and consistency of the final PVC pipes Energy efficiency: examination of ways to save material and energy use when extruding PVC pipe products
While the melting process does not provide perfect mixing, this study shows that mixing is indeed initiated during melting.
VEM Group, a global tooling and molding provider to the medical device and consumer goods industry, will exhibit at the upcoming trade fair Design & Manufacturing West, also known as MD&M West. This event is the leading U.S. tradeshow and conference for the medical device manufacturing industry which takes place August 10-12 in Anaheim, California. VEM will be exhibiting in Hall D, booth 3327.
Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Across all process types, sustainability was a big theme at NPE2024. But there was plenty to see in automation and artificial intelligence as well.
One application of the thermoset polyurethane is for sports equipment including ski boots, where impact resistance at low temperatures is desirable. Photo Credit: Chromatic 3D
In addition to winter sports gear, potential applications include parts for the aerospace, automotive and agricultural industries with varying temperature requirements.
While prices moved up for three of the five commodity resins, there was potential for a flat trajectory for the rest of the third quarter.
VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
When, how, what and why to automate — leading robotics suppliers and forward-thinking moldmakers will share their insights on automating manufacturing at collocated event.
Thousands of people visit our Supplier Guide every day to source equipment and materials. Get in front of them with a free company profile.
Mike Sepe has authored more than 25 ANTEC papers and more than 250 articles illustrating the importance of this interdisciplanary approach. In this collection, we present some of his best work during the years he has been contributing for Plastics Technology Magazine.
The aim of this presentation is to guide you through the factors and the numbers that will help you determine if a robot is a smart investment for your application. Agenda: Why are you considering automation? What problems are you trying to solve? How and why automation can help Crunch the numbers and determine the ROI
Plastics Technology covers technical and business Information for Plastics Processors in Injection Molding, Extrusion, Blow Molding, Plastic Additives, Compounding, Plastic Materials, and Resin Pricing. About Us
Formnext Chicago is an industrial additive manufacturing expo taking place April 8-10, 2025 at McCormick Place in Chicago, Illinois. Formnext Chicago is the second in a series of Formnext events in the U.S. being produced by Mesago Messe Frankfurt, AMT – The Association For Manufacturing Technology, and Gardner Business Media (our publisher).
Ultradent's entry of its Umbrella cheek retractor took home the awards for Technical Sophistication and Achievement in Economics and Efficiency at PTXPO.
Our experienced team uses the newest equipment and automation, including brands such as Arburg, Toshiba, GF, Hexagon and Makino. Being transparent when producing parts is what makes us stand out. Not only will we set up the production in accordance with all your needs but we can also help to improve process quality and reduce cycle times. In-house medical tooling VEM’s in-house medical molds have helped us develop medical parts for molding and assembly for years. Our fully owned mold manufacturer consistently gives us complete control over molds and materials, allowing for optimal quality control. Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Gifted with extraordinary technical know how and an authoritative yet plain English writing style, in this collection of articles Fattori offers his insights on a variety of molding-related topics that are bound to make your days on the production floor go a little bit better.
Across the show, sustainability ruled in new materials technology, from polyolefins and engineering resins to biobased materials.
Sustainability continues to dominate new additives technology, but upping performance is also evident. Most of the new additives have been targeted to commodity resins and particularly polyolefins.
In this collection of articles, two of the industry’s foremost authorities on screw design — Jim Frankand and Mark Spalding — offer their sage advice on screw design...what works, what doesn’t, and what to look for when things start going wrong.
Core Technology Molding turned to Mold-Masters E-Multi auxiliary injection unit to help it win a job and dramatically change its process.
Join Engel in exploring the future of battery molding technology. Discover advancements in thermoplastic composites for battery housings, innovative automation solutions and the latest in large-tonnage equipment designed for e-mobility — all with a focus on cost-efficient solutions. Agenda: Learn about cutting-edge thermoplastic composites for durable, sustainable and cost-efficient battery housings Explore advanced automation concepts for efficient and scalable production See the latest large-tonnage equipment and technology innovations for e-mobility solutions
In this collection, which is part one of a series representing some of John’s finest work, we present you with five articles that we think you will refer to time and again as you look to solve problems, cut cycle times and improve the quality of the parts you mold.
Discover how artifical intelligence is revolutionizing plastics processing. Hear from industry experts on the future impact of AI on your operations and envision a fully interconnected plant.
Exhibitors and presenters at the plastics show emphasized 3D printing as a complement and aid to more traditional production processes.
Exhibitors and presenters at the plastics show emphasized 3D printing as a complement and aid to more traditional production processes.
In this three-part collection, veteran molder and moldmaker Jim Fattori brings to bear his 40+ years of on-the-job experience and provides molders his “from the trenches” perspective on on the why, where and how of venting injection molds. Take the trial-and-error out of the molding venting process.

Being transparent when producing parts is what makes us stand out. Not only will we set up the production in accordance with all your needs but we can also help to improve process quality and reduce cycle times. In-house medical tooling VEM’s in-house medical molds have helped us develop medical parts for molding and assembly for years. Our fully owned mold manufacturer consistently gives us complete control over molds and materials, allowing for optimal quality control. Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
VEM Medical provides plastic injection molding, in-house tooling, prototyping, assembly, testing and more for medical devices, plastics and components.
VEM is an integrated partner from design assistance and design for manufacturing to finished device production and assembly. Our fully owned tool shops, cleanroom production, assembly and sub-assembly capabilities provide rich opportunities to partner with us, no matter which stage your product is in. Our experienced team uses the newest equipment and automation, including brands such as Arburg, Toshiba, GF, Hexagon and Makino. Being transparent when producing parts is what makes us stand out. Not only will we set up the production in accordance with all your needs but we can also help to improve process quality and reduce cycle times. In-house medical tooling VEM’s in-house medical molds have helped us develop medical parts for molding and assembly for years. Our fully owned mold manufacturer consistently gives us complete control over molds and materials, allowing for optimal quality control. Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Chromatic 3D Materials announced the launch of its ChromaMotive D65. ChromaMotive D65 is a rigid thermoset polyurethane formulated for reactive extrusion additive manufacturing with the company’s RX-AM platform.
Learn about sustainable scrap reprocessing—this resource offers a deep dive into everything from granulator types and options, to service tips, videos and technical articles.
Processors with sustainability goals or mandates have a number of ways to reach their goals. Biopolymers are among them.
Despite price increase nominations going into second quarter, it appeared there was potential for generally flat pricing with the exception of a major downward correction for PP.
In a time where sustainability is no longer just a buzzword, the food and beverage packaging industry is required to be at the forefront of this innovation. By adopting circular packaging processes and solutions, producers can meet regulatory requirements while also satisfying consumer demand and enhancing brand reputation. Join Husky to learn more about the broader implications of the circular economy — as well as how leading brands are leveraging this opportunity to reduce costs, increase design flexibility and boost product differentiation. Agenda: The cost and operational benefits of embracing circularity Key materials in circular packaging — including rPET and emerging bioplastics How to design a circular food and beverage package Strategies for selecting sustainable closures to future-proof packaging solutions Optimization and streamlining of production processes for enhanced efficiency How Husky Technologies can enable your sustainable success
technotrans says climate protection, energy efficiency and customization will be key discussion topics at PTXPO as it displays its protemp flow 6 ultrasonic eco and the teco cs 90t 9.1 TCUs.
Facing rising costs and import/export issues with medical device production in China, VEM Medical helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China, where costs continue to rise, to Thailand with supply chains intact.
A highly experienced team, best-in-class equipment and rigorous quality control help us deliver solutions for challenging medical tooling and molding applications. For more than 20 years, VEM has focused on customer satisfaction as our highest priority. Our clients range from small innovative companies to large multinational original equipment manufacturers (OEMs). Our production facility is certified according to ISO 9001 and ISO 13485, with a Class 100,000 (ISO 8) cleanroom. VEM Medical can produce small and precise molded parts for medical devices. Our medical assembly line is inspected by a stringent quality control team. A static 2K mixer for medical applications. VEM Medical can produce injection-molded parts for medical sub-assembly. Each of our manufactured devices must pass a testing procedure specified by the customer. Medical inserts can be molded in our clean room. We produce parts through complex medical tooling. Our team sub-assembles a tube connection for a breathing device. Many different parts can be moulded in our Class 100,000 cleanroom. Medical plastics and device manufacturing VEM is an integrated partner from design assistance and design for manufacturing to finished device production and assembly. Our fully owned tool shops, cleanroom production, assembly and sub-assembly capabilities provide rich opportunities to partner with us, no matter which stage your product is in. Our experienced team uses the newest equipment and automation, including brands such as Arburg, Toshiba, GF, Hexagon and Makino. Being transparent when producing parts is what makes us stand out. Not only will we set up the production in accordance with all your needs but we can also help to improve process quality and reduce cycle times. In-house medical tooling VEM’s in-house medical molds have helped us develop medical parts for molding and assembly for years. Our fully owned mold manufacturer consistently gives us complete control over molds and materials, allowing for optimal quality control. Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Join this webinar to explore the transformative benefits of retrofitting your existing injection molding machines (IMMs). Engel will guide you through upgrading your equipment to enhance monitoring, control and adaptability — all while integrating digital technologies. You'll learn about the latest trends in IMM retrofitting (including Euromap interfaces and plasticizing retrofits) and discover how to future-proof your machines for a competitive edge. With insights from industry experts, it'll walk you through the decision-making process, ensuring you make informed choices that drive your business forward. Agenda: Maximize the value of your current IMMs through strategic retrofitting Learn how to integrate digital technologies to enhance monitoring and control Explore the benefits of Euromap interfaces and plasticizing retrofits Understand how retrofitting can help meet new product demands and improve adaptability Discover how Engel can support your retrofitting needs, from free consultations to execution
Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
VEM Group, a global tooling and molding provider to the medical device and consumer goods industry, has been selected by Manufacturing Outlook magazine a Top 10 Injection Molding Service Companies 2021.
Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
VEM’s in-house medical molds have helped us develop medical parts for molding and assembly for years. Our fully owned mold manufacturer consistently gives us complete control over molds and materials, allowing for optimal quality control. Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Multiple speakers at Molding 2023 will address the ways simulation can impact material substitution decisions, process profitability and simplification of mold design.
Additive technology creates air pockets in film during orientation, cutting down on the amount of resin needed while boosting opacity, mechanical properties and recyclability.
Plastics Technology’s Tech Days is back! Every Tuesday in October, a series of five online presentations will be given by industry supplier around the following topics: Injection Molding — New Technologies, Efficiencies Film Extrusion — New Technologies, Efficiencies Upstream/Downstream Operations Injection Molding — Sustainability Extrusion — Compounding Coming out of NPE2024, PT identified a variety of topics, technologies and trends that are driving and shaping the evolution of plastic products manufacturing — from recycling/recyclability and energy optimization to AI-based process control and automation implementation. PT Tech Days is designed to provide a robust, curated, accessible platform through which plastics professionals can explore these trends, have direct access to subject-matter experts and develop strategies for applying solutions in their operations.
After successfully introducing a combined conference for moldmakers and injection molders in 2022, Plastics Technology and MoldMaking Technology are once again joining forces for a tooling/molding two-for-one.
VEM Medical is a full-service contract manufacturing organisation for medical plastics, devices and components. We partner with medical device companies across three continents to provide plastic injection molding, in-house tooling, prototyping, assembly, testing and more. A highly experienced team, best-in-class equipment and rigorous quality control help us deliver solutions for challenging medical tooling and molding applications. For more than 20 years, VEM has focused on customer satisfaction as our highest priority. Our clients range from small innovative companies to large multinational original equipment manufacturers (OEMs). Our production facility is certified according to ISO 9001 and ISO 13485, with a Class 100,000 (ISO 8) cleanroom. VEM Medical can produce small and precise molded parts for medical devices. Our medical assembly line is inspected by a stringent quality control team. A static 2K mixer for medical applications. VEM Medical can produce injection-molded parts for medical sub-assembly. Each of our manufactured devices must pass a testing procedure specified by the customer. Medical inserts can be molded in our clean room. We produce parts through complex medical tooling. Our team sub-assembles a tube connection for a breathing device. Many different parts can be moulded in our Class 100,000 cleanroom. Medical plastics and device manufacturing VEM is an integrated partner from design assistance and design for manufacturing to finished device production and assembly. Our fully owned tool shops, cleanroom production, assembly and sub-assembly capabilities provide rich opportunities to partner with us, no matter which stage your product is in. Our experienced team uses the newest equipment and automation, including brands such as Arburg, Toshiba, GF, Hexagon and Makino. Being transparent when producing parts is what makes us stand out. Not only will we set up the production in accordance with all your needs but we can also help to improve process quality and reduce cycle times. In-house medical tooling VEM’s in-house medical molds have helped us develop medical parts for molding and assembly for years. Our fully owned mold manufacturer consistently gives us complete control over molds and materials, allowing for optimal quality control. Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
This Knowledge Center provides an overview of the considerations needed to understand the purchase, operation, and maintenance of a process cooling system.
Second quarter started with price hikes in PE and the four volume engineering resins, but relatively stable pricing was largely expected by the quarter’s end.
VEM Europe, part of VEM Group, a global tooling and molding provider to the medical device and consumer goods industry, will exhibit at the upcoming trade fair Interplas, September 28-30. It is the UK’s leading plastics industry event, an exciting showcase for technologies, manufacturing processes and services essential to the plastics sector. VEM will be exhibiting at booth C69.
August 29-30 in Minneapolis all things injection molding and moldmaking will be happening at the Hyatt Regency — check out who’s speaking on what topics today.
VEM Medical is a full-service contract manufacturing organisation for medical plastics, devices and components. We partner with medical device companies across three continents to provide plastic injection molding, in-house tooling, prototyping, assembly, testing and more. A highly experienced team, best-in-class equipment and rigorous quality control help us deliver solutions for challenging medical tooling and molding applications. For more than 20 years, VEM has focused on customer satisfaction as our highest priority. Our clients range from small innovative companies to large multinational original equipment manufacturers (OEMs). Our production facility is certified according to ISO 9001 and ISO 13485, with a Class 100,000 (ISO 8) cleanroom. VEM Medical can produce small and precise molded parts for medical devices. Our medical assembly line is inspected by a stringent quality control team. A static 2K mixer for medical applications. VEM Medical can produce injection-molded parts for medical sub-assembly. Each of our manufactured devices must pass a testing procedure specified by the customer. Medical inserts can be molded in our clean room. We produce parts through complex medical tooling. Our team sub-assembles a tube connection for a breathing device. Many different parts can be moulded in our Class 100,000 cleanroom. Medical plastics and device manufacturing VEM is an integrated partner from design assistance and design for manufacturing to finished device production and assembly. Our fully owned tool shops, cleanroom production, assembly and sub-assembly capabilities provide rich opportunities to partner with us, no matter which stage your product is in. Our experienced team uses the newest equipment and automation, including brands such as Arburg, Toshiba, GF, Hexagon and Makino. Being transparent when producing parts is what makes us stand out. Not only will we set up the production in accordance with all your needs but we can also help to improve process quality and reduce cycle times. In-house medical tooling VEM’s in-house medical molds have helped us develop medical parts for molding and assembly for years. Our fully owned mold manufacturer consistently gives us complete control over molds and materials, allowing for optimal quality control. Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Join Wittmann for an engaging webinar on the transformative impact of manufacturing execution systems (MES) in the plastic injection molding industry. Discover how MES enhances production efficiency, quality control and real-time monitoring while also reducing downtime. It will explore the integration of MES with existing systems, emphasizing compliance and traceability for automotive and medical sectors. Learn about the latest advancements in IoT and AI technologies and how they drive innovation and continuous improvement in MES. Agenda: Overview of MES benefits What is MES? Definition, role and brief history Historical perspective and evolution Longevity and analytics Connectivity: importance, standards and integration Advantages of MES: efficiency, real-time data, traceability and cost savings Emerging technologies: IoT and AI in MES
For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
Say “manufacturing automation” and thoughts immediately go to the shop floor and specialized production equipment, robotics and material handling systems. But there is another realm of possible automation — the front office.
The Plastics Industry Association (PLASTICS) has released final figures for NPE2024: The Plastics Show (May 6-10; Orlando) that officially make it the largest ever NPE in several key metrics.
For more than 20 years, VEM has focused on customer satisfaction as our highest priority. Our clients range from small innovative companies to large multinational original equipment manufacturers (OEMs). Our production facility is certified according to ISO 9001 and ISO 13485, with a Class 100,000 (ISO 8) cleanroom. VEM Medical can produce small and precise molded parts for medical devices. Our medical assembly line is inspected by a stringent quality control team. A static 2K mixer for medical applications. VEM Medical can produce injection-molded parts for medical sub-assembly. Each of our manufactured devices must pass a testing procedure specified by the customer. Medical inserts can be molded in our clean room. We produce parts through complex medical tooling. Our team sub-assembles a tube connection for a breathing device. Many different parts can be moulded in our Class 100,000 cleanroom. Medical plastics and device manufacturing VEM is an integrated partner from design assistance and design for manufacturing to finished device production and assembly. Our fully owned tool shops, cleanroom production, assembly and sub-assembly capabilities provide rich opportunities to partner with us, no matter which stage your product is in. Our experienced team uses the newest equipment and automation, including brands such as Arburg, Toshiba, GF, Hexagon and Makino. Being transparent when producing parts is what makes us stand out. Not only will we set up the production in accordance with all your needs but we can also help to improve process quality and reduce cycle times. In-house medical tooling VEM’s in-house medical molds have helped us develop medical parts for molding and assembly for years. Our fully owned mold manufacturer consistently gives us complete control over molds and materials, allowing for optimal quality control. Optimisation, rigorous quality testing, and a professional approach to solving problems have always been our key approaches. Our molds are used worldwide by large medical companies, including Danaher Corporation, Cepheid, Foamtec Medical and Freudenberg Medical. Cleanroom molding and assembly VEM can offer services in design assistance, prototyping, production mold making, injection molding in a white room or cleanroom, component sourcing, assembly, testing and packaging. Both inside and outside our cleanroom, we have room and equipment for various sizes of injection machines. Our cleanroom is built in a modular way, which allows us to be flexible and increase size as quickly as possible according to customers’ needs. Three of our global locations include high-tech tool shops, which enable us to quickly develop molds from prototype to production, as well as perform maintenance and repairs. Manufacturing transfer Thinking about moving production out of China? Facing rising costs and import/export issues with medical device production in China, VEM helps clients with an expedited manufacturing transfer of plastic injection molding and assembly projects from China to Thailand with their supply chains intact. VEM has been manufacturing in Thailand for 13 years and medical device manufacturing project transfers are among our most popular services and can be set up and running within just a few weeks. For more information, read our case study under the ‘White Papers’ tab on this page. Medical plastics and device manufacturing in Thailand Manufacturing in Southeast Asia offers a lower price point, more flexibility, and a faster time to market than domestic manufacturing can provide. For more than 13 years, VEM has operated its wholly-owned manufacturing operation in Rayong, Thailand. The largest port in Thailand, Laem Chabang Port, is just a 30-minute drive from our facility and many large medical device and healthcare manufacturers are located nearby. There is an undeniable reason behind the growth of the medical device manufacturing industry in Thailand. The location is strategically attractive due to a skilled and educated workforce, a welcoming and open economy with a stable government, quality infrastructure, lower cost of labour and reduced import taxes, and ease of import/export of raw materials and components. About VEM Medical VEM currently has five manufacturing facilities across Asia, Europe and Central America. Our facility in Thailand is ISO 13485:2016 certified and specialises in medical device production and assembly. Our tool shop in Shenzhen, China, manufactures molds for our medical device clients worldwide.
“With ChromaMotive D65, manufacturers that are producing rigid plastic components can take advantage of RX-AM’s fast print speeds and the ability to print on textiles, plastics and metals. Now it’s possible to 3D print rigid parts at industrial volumes for everything from winter recreational equipment to aircraft interiors,” says Cora Leibig, Chromatic founder and CEO.
Take a deep dive into all of the various aspects of part quoting to ensure you’ve got all the bases—as in costs—covered before preparing your customer’s quote for services.
According to the company, the new material performs well in a range of extreme cold to elevated temperatures from -30˚C up to 120˚C, and has mechanical properties comparable to injection-molded TPU, HDPE and PP. ChromaMotive D65 is compatible with Chromatic’s RX-Flow printers, which are suitable for development work and low- to medium-volume industrial production.
Resin drying is a crucial, but often-misunderstood area. This collection includes details on why and what you need to dry, how to specify a dryer, and best practices.
Mixed in among thought leaders from leading suppliers to injection molders and mold makers at the 2023 Molding and MoldMaking conferences will be molders and toolmakers themselves.
Successfully starting or restarting an injection molding machine is less about ticking boxes on a rote checklist and more about individually assessing each processing scenario and its unique variables.
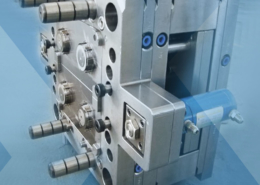
VEM Group, a global tooling and molding provider to the medical device, automotive, and consumer goods industry, has expanded operations into Eastern Europe. The wholly owned manufacturing plant is located in Plovdiv, Bulgaria, which is the cultural capital and second largest city in Bulgaria.
While prices moved up for three of the five commodity resins, there was potential for a flat trajectory for the rest of the third quarter.
GETTING A QUOTE WITH LK-MOULD IS FREE AND SIMPLE.
FIND MORE OF OUR SERVICES:


Plastic Molding
Rapid Prototyping

Pressure Die Casting

Parts Assembly



